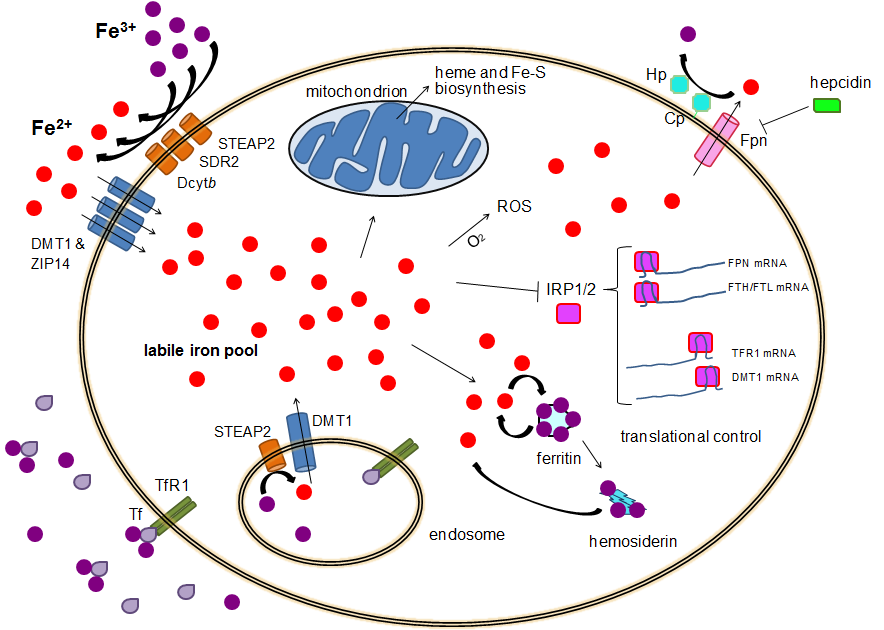
Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates - ScienceDirect

Accelerated Fe3+/Fe2+ cycle using atomic H* on Pd/Al2O3: A novel mechanism for an electrochemical system with particle electrode for iron sludge reduction in the Fe2+/peroxydisulfate oxidation process - ScienceDirect

Changes in the concentrations of DO, Fe2+, Fe3+, and Fe-precipitates... | Download Scientific Diagram

The influence of Fe2+, Fe3+ and magnet powder (Fe3O4) on aerobic granulation and their mechanisms - ScienceDirect

Changes in the concentrations of DO, Fe2+, Fe3+, and Fe-precipitates... | Download Scientific Diagram

![A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect A method for determination of [Fe3+]/[Fe2+] ratio in superparamagnetic iron oxide - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0304885317309411-gr6.jpg)